Biologics Manufacturing: The High-Stakes Battle for Pharma's Next Frontier
Manufacturing
2025-03-18 09:02:00Content
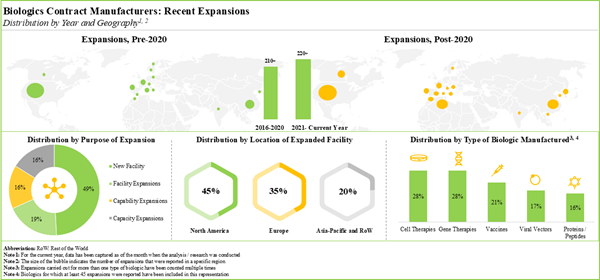
The Rising Trend of Outsourcing in Biopharmaceutical Manufacturing
The biopharmaceutical industry is experiencing a transformative shift as drug developers increasingly turn to contract manufacturers for comprehensive, end-to-end solutions. This strategic approach encompasses critical processes such as bioprocess development and optimization, signaling a significant evolution in how pharmaceutical companies approach drug production.
As outsourcing gains broader acceptance and recognition as a strategic and cost-effective business model, the global market for biologics contract manufacturing is poised for substantial growth. This trend reflects the industry's growing confidence in specialized contract manufacturing organizations that can deliver high-quality, efficient, and innovative manufacturing solutions.
The shift towards contract manufacturing is driven by several key factors, including the need for specialized expertise, reduced capital investment, increased flexibility, and the ability to accelerate time-to-market for critical biopharmaceutical products. By partnering with experienced contract manufacturers, drug developers can focus on their core competencies of research and development while leveraging external manufacturing capabilities.
Industry experts predict that this trend will continue to gain momentum, with contract manufacturing playing an increasingly pivotal role in the biopharmaceutical landscape in the coming years.
Revolutionizing Biologics: The Strategic Rise of Contract Manufacturing in Pharmaceutical Innovation
In the rapidly evolving landscape of pharmaceutical development, a transformative shift is occurring that promises to reshape how innovative therapies are brought from conceptualization to market. The pharmaceutical industry stands at a critical juncture where collaboration, efficiency, and specialized expertise are becoming paramount in delivering groundbreaking biological treatments to patients worldwide.Unleashing Potential: The Future of Pharmaceutical Manufacturing Partnerships
The Emerging Paradigm of Strategic Outsourcing
The pharmaceutical landscape is experiencing a profound metamorphosis driven by increasingly complex biological therapies. Traditional in-house manufacturing models are giving way to more dynamic, collaborative approaches that leverage specialized contract manufacturing organizations (CMOs). These strategic partnerships are not merely transactional relationships but sophisticated ecosystems of technological exchange and scientific expertise. Modern biopharmaceutical companies are recognizing that outsourcing represents more than a cost-cutting strategy—it's a sophisticated mechanism for accessing cutting-edge technological capabilities. By partnering with specialized contract manufacturers, organizations can dramatically accelerate their research and development timelines while maintaining exceptional quality standards.Technological Convergence in Bioprocess Development
Advanced contract manufacturing organizations are now offering comprehensive end-to-end solutions that transcend traditional service boundaries. These sophisticated platforms integrate multiple technological domains, including advanced bioprocess optimization, precision engineering, and regulatory compliance frameworks. The integration of artificial intelligence and machine learning technologies is revolutionizing bioprocess development. Contract manufacturers are deploying advanced predictive modeling techniques to optimize complex biological manufacturing processes, reducing development cycles and enhancing overall production efficiency. This technological convergence enables unprecedented levels of precision and reproducibility in biological drug manufacturing.Economic and Strategic Implications of Manufacturing Partnerships
The global biologics contract manufacturing market is experiencing exponential growth, driven by increasing complexity of therapeutic molecules and the need for specialized manufacturing capabilities. Pharmaceutical companies are strategically reallocating resources, recognizing that external partnerships can provide more agile and cost-effective development pathways. Economic analyses suggest that contract manufacturing can reduce capital expenditure by up to 40% compared to traditional in-house manufacturing models. These substantial cost savings are complemented by accelerated time-to-market strategies and access to specialized technological expertise that would require significant internal investment.Regulatory Landscape and Compliance Challenges
As biological therapies become increasingly sophisticated, regulatory frameworks are evolving to ensure stringent quality and safety standards. Contract manufacturers are playing a crucial role in navigating these complex regulatory environments, providing comprehensive compliance support that extends beyond traditional manufacturing services. Successful contract manufacturing partnerships require robust quality management systems, advanced traceability mechanisms, and a deep understanding of global regulatory requirements. The most effective CMOs are those that can seamlessly integrate scientific innovation with rigorous compliance protocols.Future Trajectory of Biologics Manufacturing
The future of biologics manufacturing will be characterized by unprecedented levels of technological integration, collaborative innovation, and strategic partnerships. As therapeutic approaches become more personalized and molecularly complex, the role of specialized contract manufacturers will become increasingly critical. Emerging technologies such as continuous manufacturing, advanced analytics, and adaptive process control are poised to transform the biologics manufacturing landscape. Contract manufacturers who can effectively leverage these technologies will be at the forefront of pharmaceutical innovation, driving more efficient, precise, and patient-centric therapeutic solutions.RELATED NEWS

Forging Futures: How Northeast Indiana is Transforming Student Pathways into Manufacturing Powerhouses

Manufacturing's Broken Promise: How America's Job Recovery Plan Fell Short