Breaking: Global Clinical Trial Supplies Market Set to Revolutionize Drug Development by 2029
Manufacturing
2025-04-17 09:40:00Content
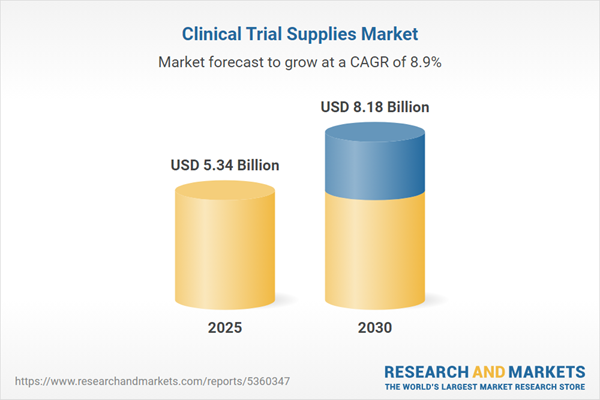
Clinical Trial Supply Chain: Driving Innovation and Efficiency in Pharmaceutical Research
The clinical trial supply chain is experiencing a transformative era, powered by a convergence of dynamic factors that are reshaping the pharmaceutical research landscape. Unprecedented growth is being fueled by three critical drivers: escalating trial complexity, the rapid expansion of personalized medicine, and increasingly stringent regulatory requirements.
Emerging Trends Revolutionizing Clinical Trial Logistics
The industry is witnessing a paradigm shift towards more sophisticated and technology-driven approaches. Key trends include:
- Decentralized clinical trials that offer greater patient flexibility
- Advanced logistics solutions with enhanced tracking and management capabilities
- Digital transformation leveraging cutting-edge technologies like Internet of Things (IoT) and Artificial Intelligence (AI)
Market Dynamics and Strategic Segments
Current market dynamics highlight the prominence of Logistics & Distribution Services and Phase III trials, with North America emerging as the dominant market leader. Pharmaceutical companies and Contract Research Organizations (CROs) are strategically deploying innovative technologies to optimize supply chain efficiency and streamline research processes.
Future Outlook
As the clinical trial supply chain continues to evolve, stakeholders are poised to embrace technological innovations that promise enhanced precision, reduced costs, and accelerated research timelines.
Revolutionizing Clinical Trials: The Tech-Driven Transformation of Medical Research
In the rapidly evolving landscape of medical research, clinical trials are undergoing a profound metamorphosis, driven by technological innovation, regulatory complexities, and the urgent need for more personalized healthcare solutions. The traditional paradigms of medical research are being systematically dismantled and reconstructed, creating an unprecedented era of scientific discovery and patient-centric approaches.Navigating the Future: Where Technology Meets Medical Innovation
The Decentralized Revolution in Clinical Research
The pharmaceutical industry is experiencing a seismic shift towards decentralized clinical trials, fundamentally reimagining how medical research is conducted. Traditional brick-and-mortar research models are giving way to more flexible, technology-enabled approaches that prioritize patient convenience and data integrity. Advanced digital platforms now allow participants to engage in studies from the comfort of their homes, dramatically expanding research accessibility and participant diversity. Cutting-edge technologies like remote monitoring devices, telemedicine platforms, and sophisticated data collection systems are breaking down geographical barriers. These innovations enable researchers to capture real-time patient data, reduce logistical complexities, and create more comprehensive and nuanced research environments that were previously unimaginable.Artificial Intelligence and the Future of Clinical Trials
Artificial Intelligence is rapidly transforming clinical trial methodologies, introducing unprecedented levels of efficiency and precision. Machine learning algorithms can now predict patient recruitment potential, optimize trial design, and identify potential risks with remarkable accuracy. These intelligent systems analyze vast datasets, uncovering patterns and insights that human researchers might overlook. The integration of AI extends beyond predictive analytics. Sophisticated algorithms are now capable of streamlining patient screening processes, matching participants with appropriate trials more effectively, and even predicting potential treatment outcomes with increasing reliability. This technological intervention is not just enhancing research capabilities but fundamentally reimagining the entire clinical trial ecosystem.Supply Chain Management in Modern Clinical Research
The logistics of clinical trials have become increasingly complex, requiring sophisticated supply chain management strategies. Internet of Things (IoT) technologies are revolutionizing how medical supplies, medications, and research materials are tracked, stored, and distributed. Real-time monitoring systems ensure temperature-sensitive medications remain within precise environmental parameters, dramatically reducing waste and improving overall research reliability. Advanced blockchain technologies are also emerging as game-changers in maintaining transparent, immutable records of clinical trial supply chains. These systems provide unprecedented levels of traceability, ensuring regulatory compliance and building trust among stakeholders.Regulatory Landscape and Technological Adaptation
Regulatory bodies are rapidly evolving to accommodate technological advancements in clinical research. Agencies like the FDA are developing more flexible frameworks that encourage innovation while maintaining rigorous safety standards. This delicate balance requires continuous dialogue between technological innovators, pharmaceutical companies, and regulatory experts. The emergence of personalized medicine is driving much of this regulatory transformation. As treatments become increasingly tailored to individual genetic profiles, clinical trials must become more nuanced and adaptable. Regulatory guidelines are being rewritten to accommodate these more complex, individualized research methodologies.Global Market Dynamics and Regional Innovations
North America continues to lead the global clinical trials market, driven by substantial research investments, advanced technological infrastructure, and a robust pharmaceutical ecosystem. However, emerging markets in Asia and Europe are rapidly developing their own innovative approaches, creating a more distributed and collaborative global research environment. The competitive landscape is characterized by strategic partnerships between pharmaceutical companies, contract research organizations (CROs), and technology providers. These collaborations are essential in pushing the boundaries of medical research and accelerating the development of groundbreaking treatments.RELATED NEWS

Pharma Giant Doubles Down: Novartis Pumps $23 Billion into US Manufacturing Amid Trade Tensions

Trade Wars and Threads: How Trump's Tariff Plan Could Unravel US Apparel Manufacturing