Kyowa Kirin Unveils $118M Biologics Facility, Signals Global Expansion Amid US Project Progress
Manufacturing
2025-04-11 14:00:52Content
.jpg)
Kyowa Kirin Expands Global Manufacturing Capabilities with Dual Biologics Facilities
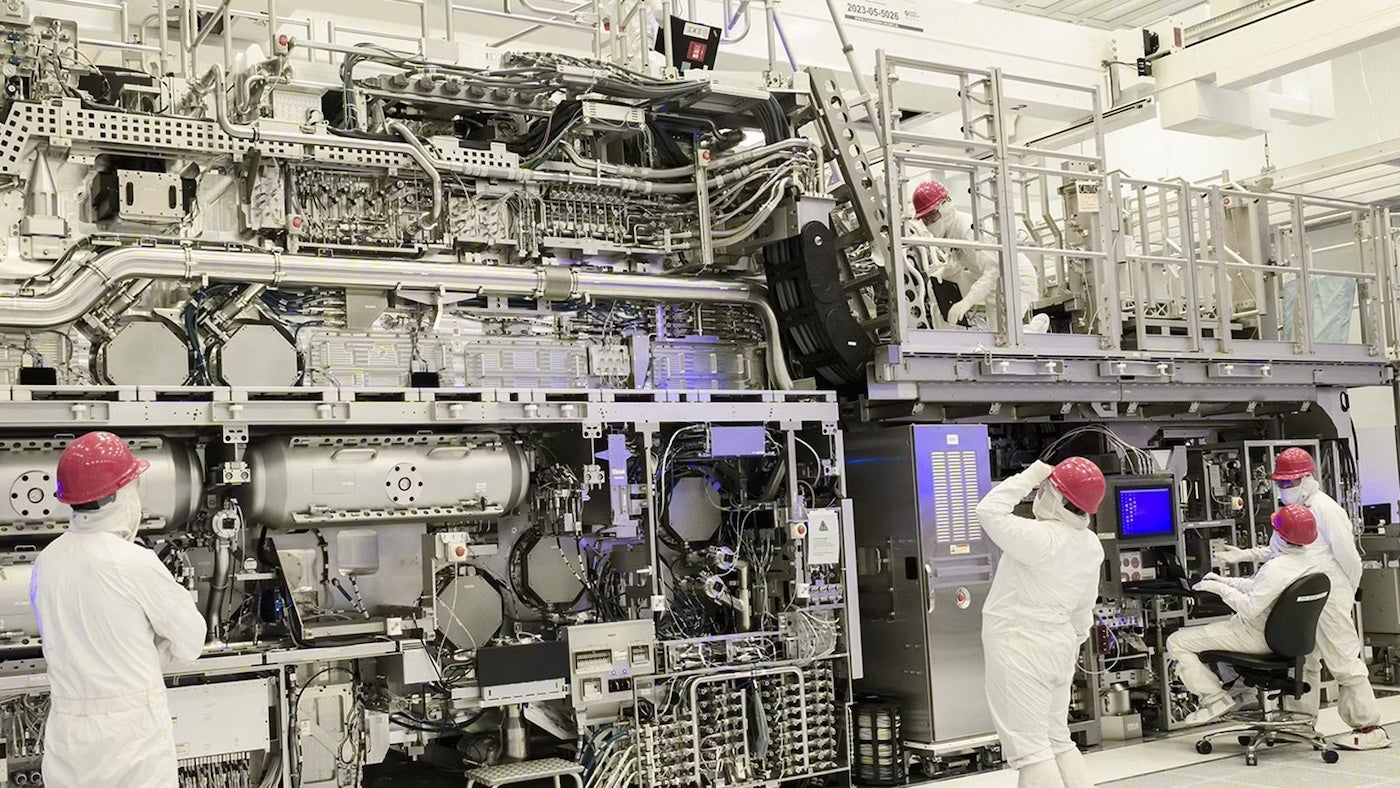
Specialty pharmaceutical company Kyowa Kirin is making significant strides in its manufacturing infrastructure, simultaneously advancing construction of a new biologics plant in North Carolina while completing a companion facility in Japan. The company's latest investment is a state-of-the-art $118 million manufacturing complex located in Takasaki, Japan, strategically designed to support early-stage drug development.
This new facility will specialize in producing investigational drugs, with a particular focus on advanced antibody research and development. By establishing these complementary manufacturing sites in both the United States and Japan, Kyowa Kirin is positioning itself to accelerate innovative biologics research and enhance its global pharmaceutical production capabilities.
The Takasaki plant represents a critical expansion of the company's research and development infrastructure, enabling more efficient and flexible drug development processes. As the pharmaceutical industry continues to evolve, Kyowa Kirin's strategic investments demonstrate its commitment to cutting-edge medical research and advanced manufacturing technologies.
Biologics Revolution: Kyowa Kirin's Strategic Global Manufacturing Expansion Unveiled
In the rapidly evolving landscape of pharmaceutical innovation, biotechnology companies are continuously pushing boundaries to develop cutting-edge therapeutic solutions. Kyowa Kirin, a pioneering specialty pharmaceutical enterprise, is making significant strides in expanding its global manufacturing capabilities through strategic investments in advanced biologics production facilities.Transforming Pharmaceutical Manufacturing: A Bold Vision for Next-Generation Drug Development
Global Infrastructure Investment Strategy
Kyowa Kirin's ambitious expansion strategy represents a sophisticated approach to pharmaceutical manufacturing that transcends traditional production models. By simultaneously developing state-of-the-art facilities in Japan and the United States, the company demonstrates a comprehensive commitment to advancing biologics research and development. These strategic investments, totaling approximately $118 million in the Takasaki facility alone, signal a profound transformation in how investigational drugs are conceptualized, developed, and brought to market. The company's multifaceted approach involves creating specialized manufacturing environments capable of producing complex biological compounds, particularly monoclonal antibodies and other advanced therapeutic molecules. These facilities are not merely production centers but represent sophisticated research ecosystems designed to accelerate drug discovery and development processes.Technological Innovation in Biologics Production
Modern biologics manufacturing demands unprecedented levels of precision, technological sophistication, and regulatory compliance. Kyowa Kirin's new facilities embody this complexity, integrating advanced bioprocessing technologies that enable more efficient, scalable, and high-quality drug production. The Takasaki plant, in particular, represents a significant leap forward in the company's capability to support early-phase drug development. By investing in cutting-edge infrastructure, Kyowa Kirin demonstrates its commitment to pushing the boundaries of pharmaceutical research. The facility's design incorporates advanced containment systems, precision manufacturing equipment, and robust quality control mechanisms that ensure the highest standards of drug development.Strategic Geographic Positioning
The simultaneous development of manufacturing facilities in Japan and North Carolina reflects a nuanced understanding of global pharmaceutical markets. This geographic diversification provides Kyowa Kirin with multiple strategic advantages, including enhanced supply chain resilience, reduced geopolitical risks, and improved access to diverse talent pools and research ecosystems. Each facility is strategically positioned to leverage local scientific expertise, regulatory frameworks, and research infrastructure. The North American facility, for instance, benefits from the region's robust biotechnology ecosystem, while the Japanese plant capitalizes on the country's renowned precision engineering and technological innovation.Future of Investigational Drug Development
Kyowa Kirin's expansion represents more than a mere infrastructure investment; it symbolizes a broader transformation in pharmaceutical research and development. By creating dedicated spaces for early-phase drug investigation, the company is positioning itself at the forefront of biologics innovation. The ability to rapidly prototype, test, and scale investigational drugs is becoming increasingly critical in an era of personalized medicine and targeted therapies. Kyowa Kirin's new facilities are designed to accelerate this process, providing researchers with the tools and environment necessary to explore groundbreaking therapeutic approaches.Economic and Scientific Implications
These strategic investments have far-reaching implications beyond the pharmaceutical industry. They represent significant economic contributions, creating high-skilled jobs, driving technological innovation, and potentially revolutionizing medical treatment paradigms. The approximately $118 million investment in the Takasaki facility alone demonstrates the substantial financial commitment required to advance biological research. Such investments not only support immediate research objectives but also contribute to long-term scientific and economic development.RELATED NEWS
Manufacturing

Manufacturing Momentum: India's Industrial Sector Roars Back with Robust Export Surge
2025-05-02 05:03:43
Manufacturing

Supply Chain Revolution: Manufacturing Sector Poised to Explode to $52.4 Billion by 2032
2025-04-08 14:16:00
Manufacturing

Green Power Revolution: California's Bold Plan to Supercharge Grid Manufacturing
2025-05-03 12:00:59